马来酸 [顺丁烯二酸],Maleic acid [Maleinic acid];electronic grade, ≥99.97% trace metals basis, water <=350 ppm
最近浏览商品
-
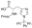 N-Fmoc-N'-三苯甲基-L-组氨酸,Fmoc-His(Trt)-OH;≥98.0% (sum of enantiomers, HPLC)
N-Fmoc-N'-三苯甲基-L-组氨酸,Fmoc-His(Trt)-OH;≥98.0% (sum of enantiomers, HPLC) -
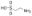 牛磺酸,Taurine;suitable for cell culture, meets USP testing specifications, 98.5-101.5%
牛磺酸,Taurine;suitable for cell culture, meets USP testing specifications, 98.5-101.5% -
![显示明细 胰蛋白酶来源于猪胰腺 [胰酶],Trypsin from porcine pancreas;lyophilized powder, Type II-S, 1,000-2,000 units/mg dry solid 图片 胰蛋白酶来源于猪胰腺 [胰酶],Trypsin from porcine pancreas;lyophilized powder, Type II-S, 1,000-2,000 units/mg dry solid](https://www.bszh.cn/images/thumbs/0014597_-trypsin-from-porcine-pancreaslyophilized-powder-type-ii-s-1000-2000-unitsmg-dry-solid_32.jpeg) 胰蛋白酶来源于猪胰腺 [胰酶],Trypsin from porcine pancreas;lyophilized powder, Type II-S, 1,000-2,000 units/mg dry solid
胰蛋白酶来源于猪胰腺 [胰酶],Trypsin from porcine pancreas;lyophilized powder, Type II-S, 1,000-2,000 units/mg dry solid -
![显示明细 β-甘油磷酸二钠盐 [β-甘油磷酸钠],β-Glycerophosphate disodium [BGP];≤1.0 mol % L-α-isomer 图片 β-甘油磷酸二钠盐 [β-甘油磷酸钠],β-Glycerophosphate disodium [BGP];≤1.0 mol % L-α-isomer](https://www.bszh.cn/images/thumbs/0004369_-glycerophosphate-disodium-bgp10-mol-l-isomer_32.png) β-甘油磷酸二钠盐 [β-甘油磷酸钠],β-Glycerophosphate disodium [BGP];≤1.0 mol % L-α-isomer
β-甘油磷酸二钠盐 [β-甘油磷酸钠],β-Glycerophosphate disodium [BGP];≤1.0 mol % L-α-isomer -
 用于CVD薄膜生长的纳米金刚石晶种,CVD Seeding Suspensions;Grey Seeds
用于CVD薄膜生长的纳米金刚石晶种,CVD Seeding Suspensions;Grey Seeds -
 磷酸葡萄糖异构酶来源于兔肌肉,Phosphoglucose Isomerase from rabbit muscle;Type XI, lyophilized powder, ≥200 units/mg protein
磷酸葡萄糖异构酶来源于兔肌肉,Phosphoglucose Isomerase from rabbit muscle;Type XI, lyophilized powder, ≥200 units/mg protein

